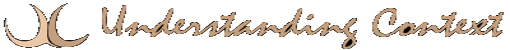27 Dec Microtubules
Microtubules
Neurons have hundreds of MT distributed in the soma and in the axon and dendrites. Microtubules, like IF, are filamentous organelles that form the cytoskeleton of neurons. Their cylindrical, composite polymers comprise part of the cytoskeletal infrastructure of cells. Their cylinders are long, and they possess a distinct polarity. Their surfaces are composed of spherical tubulin in columns called protofilaments (see illustrations below). Protofilaments are made up of pairs of alpha- and beta-tubulin dimers.
Search the web for images of Microtubules (MT). The sources contain fascinating information and excellent images. The one I borrowed at right is from UConn School of Medicine, Farmington, CT and MBL Summer Physiology Course, Class of 2010, Woods Hole, MA. Alex Lomakin. Neuronal MTs have 13 protofilaments each. It is unclear whether the last of the 13 protofilaments has the same tubulin composition as the rest, or whether it has a distinct molecular makeup. If it is different, it would be considered the “seam” of the tubule [Linck & Langevin, 1982]. There is also some possibility that it could serve as an insulator to channel electrical propagation along the MT surface, or it may just serve as a fastener or guide during fabrication.
The tubulin subunits are roughly four nanometers in diameter. The complexity of MT falls somewhere between that of ribosomes and mitochondria, both of which perform complex synthesis processes.
| Understanding Context Cross-Reference |
|---|
| Click on these Links to other posts and glossary/bibliography references |
|
|
|
| Prior Post | Next Post |
| Cytoskeleton Components in Cognition | Microtubule Functions |
| Definitions | References |
| cytoskeleton | Photo Contest MT Winner |
| Microtubules | Linck & Langevin 1982 |
| microtubule organizing centers | Linck 1989 |
MT Life Cycle
Microtubules are generally more transient than intermediate filaments. Put another way, the turn-over rates for IF are orders of magnitude more stable. When exposed to cold temperatures, MT will disassemble. Because MT are capable of replicating all or part of their structure as a new tubule, the impact of the turnover rates is minimized. In cases where MT replicate part of the tubule, they are capable of fusing old and new sections into a complete tubule. The turnover rate and the ability to self-replicate are important considerations in theories of human data storage (memory).
MT can range in length from ~0.15 micrometers to 12 mm or more. The length is not random; in fact, it is important in MT functions. The determination of length is cell specific, and it is precisely fixed within cells. Neuronal MTs are found throughout the length of cell processes (axons and dendrites) and around the nucleus in the soma. They are fixed or attached at the soma end of the process to the basal body. Some MT may extend through most of the length of the processes, while others are shorter.
Tubulin Building Blocks
Tubulin dimers, the primary components of MT, have a complexity all their own. Close examination of the possible contributions of the tubulin building blocks needs to be part of any consideration of the potential roles of MT in information processing and storage.
MT are formed from alpha- and beta-tubulin subunits. In the illustrations, this dichotomy is represented by red and blue spheres. The fusion of the two dimers actually looks more like the illustration at left than the distinct spheres shown in most of the other MT illustrations. You will notice that MT have a profound symmetry because of the simple dichotomy of the building blocks.
Together, alpha and beta tubulin form heterodimer dimers. Stable MT can possess multiple compositions or isoforms, either from the same or different MT genes, even in the same tubule. This variability is an important characteristic if the tubulin is to be considered as a storage or processing component in the neuron. Also important is the fact that the states of individual tubulin in MT can be altered even after the MT are formed.
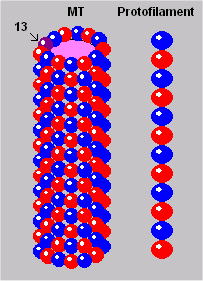
MT Lattice
 The outer structure of the MT cylinder resembles an array or lattice. Think of one of the protofilaments as a seam, along which the tubule could be disconnected and flattened out. The resulting lattice of tubulin dimers is represented in the illustration to your left.
The outer structure of the MT cylinder resembles an array or lattice. Think of one of the protofilaments as a seam, along which the tubule could be disconnected and flattened out. The resulting lattice of tubulin dimers is represented in the illustration to your left.
Fabrication of the elaborate microtubule arrays is initiated by microtubule organizing centers (MTOCs). It is not yet understood how these cell-specific and species-specific patterns are generated. Still, we do know from Linck’s research that the “number of protofilaments in MT and the nature of intertubule bridges and MTOCs are major factors” (1989, p.14). The content of MT cylinders (lumens) has not been clearly established.
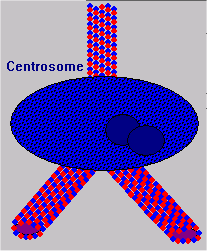
Microtubule Formation
Microtubule organizing centers (MTOC) are located in centrosomes. In fact, MTs radiate from the centrosome throughout the cell formation process. As new MTs grow, their tubulin dimers are formed in the MTOC and organized into the lattice structure. 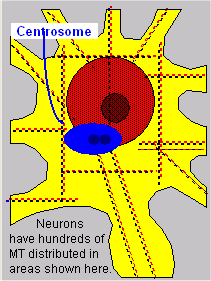 This process continues in mature cells as well, during the formation of neurites or new axon and dendrite fibers. As new fibers grow, the cytoskeleton provides structure to keep them in place and to propel them to their targets.
This process continues in mature cells as well, during the formation of neurites or new axon and dendrite fibers. As new fibers grow, the cytoskeleton provides structure to keep them in place and to propel them to their targets.
The lattice may be formed on the basis of some template either in the genes or in some specialized organelle. Basal bodies have been identified as providing templates for MT array synthesis, and centrioles have been identified for determining the exact number of protofilaments (Linck, 1989, p. 18).
Both the number of protofilaments and the arrangement of tubulin in the lattice appear to play major roles in governing MT functionality. One functional characteristic affected by the number of protofilaments is the bond angle of intermicrotubule bridges. This, in turn, governs the long-range order of MT organization within cells.
Click this link to see an MT fabrication video.
Molecular Structure
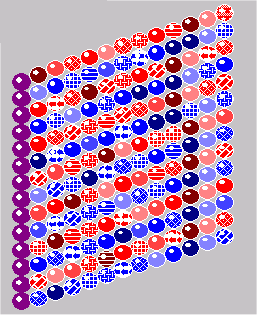 With 17 different isoforms, a single MT lattice could possess a structure as complex as that illustrated here. Individual MT probably have a simpler structure than this with fewer isozymes. Still, the possibilities for functions similar to computer logic gates or memory arrays would only require a couple of different isozymes with different electrical properties to be able to act like components of a digital computer. McCulloch and Pitts suggested the idea of Neurons as Logic Gates back in the early 1940s.
With 17 different isoforms, a single MT lattice could possess a structure as complex as that illustrated here. Individual MT probably have a simpler structure than this with fewer isozymes. Still, the possibilities for functions similar to computer logic gates or memory arrays would only require a couple of different isozymes with different electrical properties to be able to act like components of a digital computer. McCulloch and Pitts suggested the idea of Neurons as Logic Gates back in the early 1940s.
The molecular structure of tubulin dimers is polar; they naturally bind to alpha or beta counterparts. Richard Linck, a leading researcher in MT structure explains: “Built into the structure of tubulin is the information to assemble into protofilaments in a polar fashion and to assemble laterally to form cylindrical sheets or tubules” (1989, p. 16). Besides the basic isoforms of tubulin, at least three types of modified alpha and beta subunits are found in MT: phosphorylated, tyrosinated, and acetylated. All together, 17 different isozymes have been associated with MT.
MT Polarity
With such diversity, it appears that the arrangement of tubulin dimers in the microtubule wall can have major consequences on microtubule function, including the “assembly, disassembly, stability, and interactions with effector microtubule associated proteins” (Linck, 1989, p. 17). Magnesium, salt, and heat must be present for the assembly of microtubules; calcium, on the other hand, inhibits their assembly. The MT lattice is formed on the basis of a template. It has been demonstrated that MT can be templated by both MT genes and by existing MT.
Microtubules also have a definite polarity and parallel microtubules within a functioning unit, such as an axon or dendrite. These share a common polarity. The most commonly observed polarity in MTs is the negative terminal proximal (nearer the cell body) and the positive terminal distal (away from the soma). As Hameroff and Watt point out, the “transfer of excited electron resonance energy has been demonstrated between MT and membrane proteins” (1982, p. 553).
If the E/I flow is in the membrane, it is likely that all flow is identical. If that is the case, changes in intensity (weight) would only occur at the synapse. If, however, action potential flows in the cytoplasm of cells instead of the membrane, the cytoskeleton could provide pathways like wires. If that is the case, IF and MT structure could modify electrical potentials before they reach the synapses.
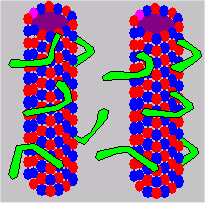 MAPs
MAPs
Certain microtubule-associated proteins (MAPs) tend to bind to the tubulin exterior of MT. Dynein and kinesin are the two primary proteins associated with MT. Dynein is a high-molecular weight MAP which forms radial spokes or arms on the surface of MT. These dynein arms appear to be quite symmetrical, occurring at intervals of 24 nm along the axis of the tubule.
The A end of dynein molecules forms stable (adenosine-triphosphate or ATP independent) connections with microtubules. The B end of the dynein molecule forms a transient connection or cross bridge to an adjacent microtubule subfiber. The mechanism for determining dynein binding sites is not known, but the exact loci of the sites may have functional significance.
There are three functional categories of MAPs: structural, force-producing, and generic. The navigational functions of MT are probably assisted by the force-producing MAPs. Dynein and kinesin are generally classified as force-producing MAPs (sometimes called mechano-chemical translocators). Guanine nucleotide is a structural MAP essential in the final assembly of MT. It may also assist in determining isozymes.
Why are MAPs interesting? MAPs may help MTs twist or bend to bring them closer or further from the cell membranes.
| Click below to look in each Understanding Context section |
|---|